APM streamlined their deviation process to improve efficiency, real-time-reporting and deeply engage operators in the root cause of compliance issues. A review of their strategy execution, along with implementation of TeamAssurance, enabled APM to achieve the following results:
- Site-wide visibility of deviation progress and status
- Reduced escalations to leadership
- Reduced the cycle time of deviations
- Reduced the deviation backlog

Cross-team communication delays
APM (Australian Pharmaceutical Manufacturers) is one of the largest providers of manufacturing and packaging services for nutraceuticals in Australia, and count many of the leading participants in the health and wellness sector as customers.
Deviations were previously managed on a hard copy form. This made it difficult to track who had the physical document, and at what stage the investigation was up to. Also as it was a hand written document, legibility was challenging at times. It was often not completed until after all actions had been finished, relying a lot on peoples memory of decisions that were made.
Actions and escalations were written on post-it notes and added to a team’s board. Context to help and track completion was definitely challenging with this system. The information captured and shared in tier meetings was siloed and hard to access. Status was unclear, actions were sometimes ‘parked’ resulting in a deviation backlog. Escalations required moving the post-it from one team’s board to another. As a result only the major escalations were being moved consistently and it was not always easy to find the post-it for an action.
Real-time reporting of compliance deviations
The Quality team took their hardcopy deviation form and designed it as a TeamAssurance Checklist. They made the checklist available to Operators, confident that they would raise deviations because it’s the same platform they’re using to manage their day-to-day activities. The increase in engagement from the frontline and improved cross-team collaboration has reduced the compliance administration effort on the Quality team. Actions travel together with the deviation, and anyone that is assigned an action has context through the details, photos, videos and comments. No more running around the hallways and trying to find and decipher the latest developments.
Effective delegation leads to better planning
Previously there was a deviation backlog and there were logistic issues related to stocking product that was waiting to be shipped. These days it is rare for the deviation backlog to hold back a batch release.
The Leadership Team have real-time visibility of all deviations, and updates of status, accountability and due dates can be changed on the digital board. The Leadership team are also less impacted by deviations, because escalations are now an exception rather than the rule.
“We were having a lot of delays with actually getting deviations raised. They would come up as escalation – say to all the way up to leadership team level, then the decision was made to raise a deviation. Why hasn’t a deviation already being raised? And that question was getting asked a lot. Now we’re getting things raised straightaway by all levels of the company“
– Alison Rose, Quality Manager
Previously the Quality team could see out about 2 weeks and were reacting to post-it notes. Now that they have visibility well beyond the next fortnight. They can prioritise and plan deviation work to effectively manage the release schedule.
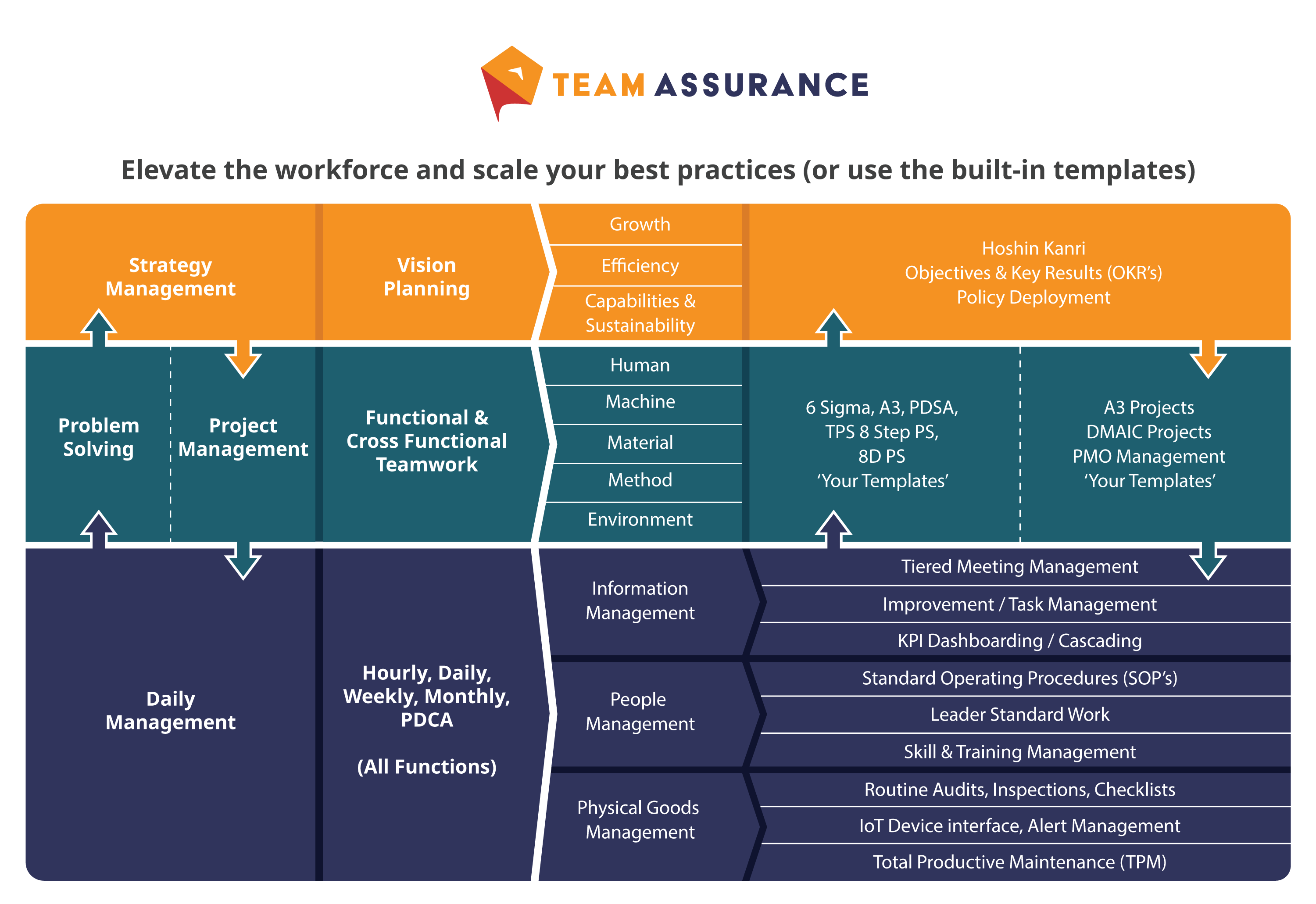
Compliance is Just One Part of the C.I. Framework, Not Just Locally Optimised ‘Islands’
For any of the above processes to not only be sustained, but to thrive, they must be supported by adjacent tools and systems. Organisations must keep in mind how the various elements interconnect – and ensure they don’t optimise or develop them in isolation.
Standardised problem solving techniques, clear documentation / audit trails, visual management and a Tiered Daily Management system should all integrate to handle the full PDCA loop.
The image below of the TeamAssurance platform below shows how we designed an interconnected platform that avoids disconnected ‘Point Solutions’ (digital or analog) that do not help, and or even act as barriers, to achieving an optimised CI framework.
 Want to explore the opportunities offered by digital-aids to Lean tools? If you’re a Business in need, or a Consultant with clients in need, contact us for a demonstration of the TeamAssurance platform today.
Want to explore the opportunities offered by digital-aids to Lean tools? If you’re a Business in need, or a Consultant with clients in need, contact us for a demonstration of the TeamAssurance platform today.